Abstract
Introduction: Idecabtagene vicleucel (ide-cel, bb2121) is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell therapy approved in the US by the Food and Drug Administration (FDA) for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody, i.e. triple-class exposed (TCE).
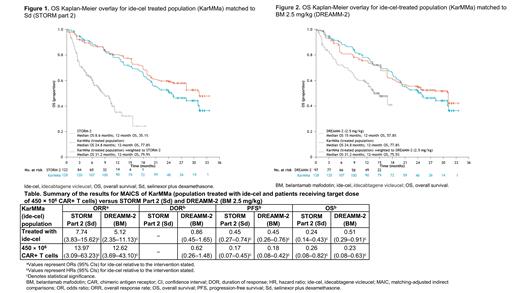
Selinexor plus dexamethasone (Sd) and belantamab mafodotin (BM) are 2 other FDA-approved regimens for similarly heavily pretreated patients, including those who are TCE. All 3 interventions were evaluated in phase 2 single-arm clinical trials: KarMMa (ide-cel), STORM Part 2 (Sd), and DREAMM-2 (BM). Previously, efficacy outcomes were compared between ide-cel (median follow-up: 13.3 months) and Sd (median follow-up: not reported [NR]) or BM (median follow-up: 6.3 months) using unanchored matching-adjusted indirect comparisons (MAICs) (Rodríguez-Otero P, et al. HemaSphere 2020;4(Suppl 2). Abstract EP969.). The current analysis updates the MAICs using longer follow-up data for ide-cel (median: 19.9 months) and BM (median: 9.0 months).
Methods: Between-study differences in patient characteristics were adjusted using individual-level patient data (IPD) from KarMMa and aggregate-level data from either STORM Part 2 or DREAMM-2. The populations of interest included the population treated with ide-cel (N = 128) from KarMMa (of whom 54 patients received the 450 × 10 6 CAR+ T cell target dose), the modified intention-to-treat (ITT) population from STORM Part 2 (N = 122), and the ITT population for the 2.5 mg/kg dose of BM from DREAMM-2 (N = 97). A weighted logistic regression was performed to calculate odds ratios (ORs) for binary outcomes and a weighed Cox regression was performed to calculate hazard ratios (HRs) for time-to-event outcomes. Outcomes of interest included overall response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). A Kaplan-Meier curve for DOR was NR in STORM Part 2; therefore, the DOR comparison was restricted to DREAMM-2. Selection of covariates for the propensity model was informed by a literature review that identified prognostic factors included in published indirect treatment comparisons involving RRMM trials, a previous analysis comparing the KarMMa patient cohort treated with ide-cel to a real-world cohort, and clinical consultation.
Results: Based on the KarMMa population treated with ide-cel, ide-cel was associated with an improvement in ORR versus Sd (OR, 7.74 [95% confidence interval (CI), 3.83-15.62]) and BM (OR, 5.12 [95% CI, 2.35-11.13]) (Table). DOR was comparable between ide-cel and BM (HR, 0.86 [95% CI, 0.45-1.65]), while ide-cel extended PFS versus Sd (HR, 0.45 [95% CI, 0.27-0.74]) and BM (HR, 0.45 [95% CI, 0.26-0.76]). Similarly, ide-cel extended OS versus Sd (HR, 0.24 [95% CI, 0.14-0.43]) and BM (HR, 0.51 [95% CI, 0.29-0.91]) (Figures 1 and 2). Results also demonstrated that ide-cel 450 × 10 6 CAR+ T cell target dose was more efficacious than Sd and BM; ide-cel resulted in an improved ORR versus Sd (OR, 13.97 [95% CI, 3.09-63.23]) and BM (OR, 12.62 [95% CI, 3.69-43.10]), and extended PFS versus Sd (HR, 0.17 [95% CI, 0.07-0.45]) and BM (HR, 0.18 [95% CI, 0.08-0.42]), and OS versus Sd (HR, 0.26 [95% CI, 0.08-0.82]) and BM (HR, 0.23 [95% CI, 0.08-0.63]). Based on patients treated with the 450 × 10 6 CAR+ T cell target dose from KarMMa, DOR was comparable between ide-cel and BM (HR, 0.62 [95% CI, 0.26-1.48]).
Conclusions: Longer follow-up data from KarMMa reported deep and durable responses, confirming earlier findings that suggest that ide-cel offers clinically meaningful benefits in ORR, PFS, and OS over other approved regimens for patients with heavily pretreated RRMM. While every attempt was made to ensure a robust approach to the selection of prognostic factors included in the propensity model, it is challenging to quantify the extent of residual bias in the treatment effect estimates due to the absence of IPD for STORM Part 2 and DREAMM-2 and any unmeasured confounders.
Rodríguez-Otero: Regeneron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy; Janssen, Celgene, Amgen, Oncopeptides, Sanofi, Abbvie, GlaxoSmithKline, Kite Pharma: Consultancy, Honoraria, Speakers Bureau. Mojebi: PRECISIONheor: Current Employment. Ayers: PRECISIONheor: Current Employment. Dhanda: BMS: Current Employment, Current equity holder in publicly-traded company. Farrell: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Davies: Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Constellation: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Delforge: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Weisel: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; Novartis: Honoraria; Pfizer: Honoraria. Towle: PRECISIONheor: Current Employment. Marshall: Bristol Myers Squibb: Current Employment. Caisip: PRECISIONheor: Current Employment. Cope: PRECISIONheor: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal